- Huntington’s Disease: A Comprehensive Overview
- Introduction
- Etiology and Genetic Basis
- Pathophysiology
- Clinical Manifestations
- Diagnosis
- Management
- Research and Future Directions
- Living with Huntington’s Disease
- Support and Resources
- Conclusion
- External Links
- Impaired Communication: Reduced Neurovascular Unit Phase Coherence in Huntington’s Disease
- Internal Brain Article References
Huntington’s Disease: A Comprehensive Overview
Introduction
Huntington’s disease (HD) is a progressive, genetic neurodegenerative disorder characterized by motor dysfunction, cognitive decline, and psychiatric disturbances. The disease is caused by a mutation in the HTT gene, leading to the production of an abnormal form of the huntingtin protein. This protein aggregation in brain cells leads to their dysfunction and eventual death.
Etiology and Genetic Basis
Huntington’s disease is inherited in an autosomal dominant pattern, meaning that a single copy of the mutated gene from an affected parent can cause the disease. Each child of an affected parent has a 50% chance of inheriting the mutation. The mutation involves an expanded CAG trinucleotide repeat in the HTT gene, with a higher number of repeats correlating with an earlier onset of symptoms.
Pathophysiology
The huntingtin protein, produced by the HTT gene, is essential for neuronal function. In HD, the mutant huntingtin protein forms aggregates that disrupt cellular processes, including protein degradation, mitochondrial function, and transcription regulation. This leads to neuronal dysfunction and death, particularly in the basal ganglia, a brain region crucial for motor control. The progressive neurodegeneration also affects other brain regions, including the cortex, which contributes to the cognitive and psychiatric symptoms observed in patients.
Clinical Manifestations
Huntington’s disease typically presents in mid-adulthood, though juvenile forms also exist. Symptoms are categorized into motor, cognitive, and psychiatric domains:
- Motor Symptoms: Chorea (involuntary, jerky movements), dystonia, bradykinesia, and impaired coordination. As the disease progresses, motor function declines, leading to severe difficulties in walking, swallowing, and speaking.
- Cognitive Symptoms: Gradual decline in cognitive abilities, including executive functions, memory, and attention. This often progresses to dementia in the later stages. Cognitive impairments significantly impact daily functioning and independence.
- Psychiatric Symptoms: Depression, anxiety, irritability, and obsessive-compulsive behaviors are common. Suicidal tendencies and psychosis may also occur. The psychiatric symptoms can often precede motor symptoms, making early diagnosis challenging.
Diagnosis
Diagnosis is primarily based on clinical presentation and family history, supported by genetic testing to identify the HTT gene mutation. Predictive genetic testing is available for at-risk individuals, particularly those with a family history of HD. Pre-symptomatic testing can help individuals make informed decisions about family planning and career choices.
Management
While there is no cure for Huntington’s disease, treatment focuses on managing symptoms and improving quality of life. A multidisciplinary approach is often required, involving neurologists, psychiatrists, physical therapists, occupational therapists, and speech therapists.
Pharmacological Treatments:
- Motor Symptoms: Tetrabenazine and deutetrabenazine are commonly used to control chorea. Antipsychotic medications like haloperidol can help manage movement and psychiatric symptoms but require monitoring for side effects.
- Psychiatric Symptoms: Antidepressants such as fluoxetine and sertraline, antipsychotics like risperidone and olanzapine, and mood stabilizers like lithium are prescribed based on individual patient needs.
Non-Pharmacological Treatments:
- Physical Therapy: Exercises to maintain mobility and balance, reducing the risk of falls. Physical therapy also focuses on strength training to preserve muscle function and joint flexibility.
- Occupational Therapy: Techniques and devices to aid in daily activities and maintain independence, such as adaptive utensils for eating and modified clothing for dressing.
- Speech Therapy: Interventions to address speech and swallowing difficulties, including exercises to strengthen the muscles involved in speech and swallowing, and the use of communication aids.
- Psychotherapy: Helps manage emotional and behavioral aspects of the condition. It provides coping strategies for patients and their families and addresses issues such as depression, anxiety, and social withdrawal.
Research and Future Directions
Ongoing research aims to better understand the molecular mechanisms of HD and develop disease-modifying therapies. Promising areas include:
- Investigating the role of the huntingtin protein in cell signaling and DNA repair. Studies are examining how modifications to the huntingtin gene during development and adulthood affect disease onset and progression.
- Developing biomarkers for early diagnosis and progression monitoring. Biomarkers can help identify the disease before significant symptoms appear and track its progression more accurately.
- Exploring gene silencing techniques, such as antisense oligonucleotides, to reduce the production of the mutant huntingtin protein. These experimental treatments aim to slow down or halt disease progression by targeting the root cause of HD.
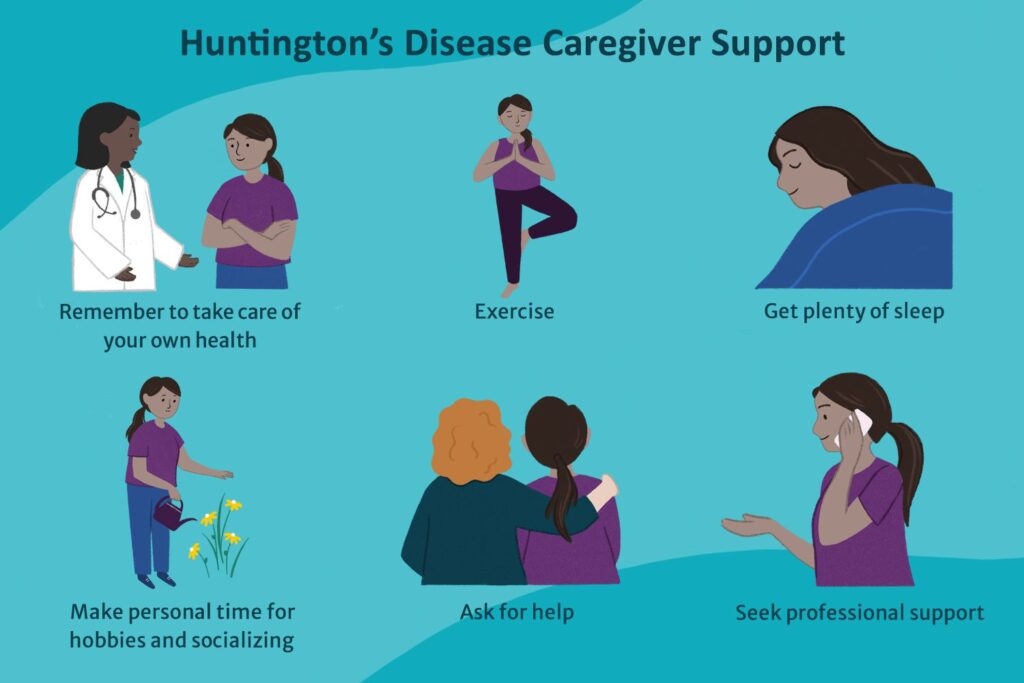
Living with Huntington’s Disease
Living with HD involves coping with progressive disability and the need for increasing levels of care. Support from healthcare professionals, caregivers, and community resources is vital. Maintaining physical activity and social engagement can improve outcomes and quality of life. Programs and support groups offered by organizations such as the Huntington’s Disease Society of America (HDSA) provide valuable resources for patients and their families.
Support and Resources
Several organizations provide support and resources for individuals and families affected by Huntington’s disease:
- Huntington’s Disease Society of America (HDSA): Offers support groups, educational resources, and advocacy.
- National Institute of Neurological Disorders and Stroke (NINDS): Provides information on current research and clinical trials.
- Mayo Clinic: Offers comprehensive care and detailed information on managing HD.
Conclusion
Huntington’s disease presents significant challenges due to its progressive and multifaceted nature. Advances in research offer hope for future therapies that may alter the course of the disease. In the meantime, a multidisciplinary approach to care can help manage symptoms and support affected individuals and their families.
By understanding the genetic basis, clinical manifestations, and current management strategies, we can better support those affected by Huntington’s disease and contribute to ongoing efforts in research and care.
External Links
- National Institute of Neurological Disorders and Stroke (NINDS) – Comprehensive resource on HD research and clinical trials.
- Huntington’s Disease Society of America (HDSA) – Support groups, educational resources, and advocacy for HD patients and families.
- Mayo Clinic – Detailed information on managing Huntington’s disease and comprehensive care options.
Impaired Communication: Reduced Neurovascular Unit Phase Coherence in Huntington’s Disease
Abstract
Huntington’s disease (HD) is a progressive neurodegenerative disorder marked by motor dysfunction, cognitive decline, and psychiatric symptoms. Recent research suggests that neurovascular dysfunction, particularly reduced phase coherence in the neurovascular unit (NVU), may play a critical role in the pathophysiology of HD. This article reviews the current understanding of NVU phase coherence, its impairment in HD, and potential implications for disease progression and treatment strategies.
Introduction
Huntington’s disease (HD) is caused by a genetic mutation leading to the production of an abnormal huntingtin protein, which aggregates in neurons and causes widespread neuronal dysfunction and death. While much research has focused on the neuronal aspects of HD, emerging evidence indicates significant involvement of neurovascular components. The neurovascular unit (NVU), comprising endothelial cells, pericytes, astrocytes, neurons, and extracellular matrix components, is essential for maintaining cerebral blood flow and the blood-brain barrier (BBB). This review explores how impaired NVU phase coherence contributes to HD pathology and examines potential therapeutic avenues.
The Neurovascular Unit: Structure and Function
The NVU is a complex system that ensures proper neuronal function by regulating blood flow, maintaining the BBB, and facilitating nutrient and waste exchange. It involves close communication between vascular cells (endothelial cells and pericytes) and neural cells (astrocytes, microglia, and neurons). NVU phase coherence refers to the synchronized activity and functional connectivity among these components, which is critical for cerebral homeostasis.
Phase Coherence in the NVU
Phase coherence in the NVU involves the temporal alignment of oscillatory activities between neurons and vascular cells. This alignment is essential for the neurovascular coupling (NVC) process, where neural activity leads to corresponding changes in cerebral blood flow. Disruptions in phase coherence can impair this coupling, leading to inadequate blood supply during neural activity and contributing to neurodegeneration.
Impairment of NVU Phase Coherence in Huntington’s Disease
Studies have shown that HD is associated with significant NVU dysfunction. The following mechanisms are implicated in the reduced phase coherence observed in HD:
- Endothelial Dysfunction: The integrity of the endothelial cells forming the BBB is compromised in HD, leading to increased permeability and reduced phase coherence with neural activity.
- Astrocyte Dysfunction: Astrocytes play a crucial role in NVC by releasing vasoactive substances in response to neuronal activity. In HD, astrocyte function is impaired, disrupting their ability to regulate blood flow efficiently.
- Pericyte Loss: Pericytes regulate capillary blood flow and maintain BBB integrity. HD pathology includes a reduction in pericyte numbers, which correlates with impaired NVC and phase coherence.
- Inflammatory Responses: Chronic neuroinflammation in HD can alter NVU function, contributing to reduced phase coherence and exacerbating neuronal damage.
Experimental Evidence
Research involving HD models has demonstrated reduced NVU phase coherence using various imaging and electrophysiological techniques. Functional MRI studies reveal abnormal cerebral blood flow patterns, while electrophysiological recordings show desynchronized neural and vascular activities.
Clinical Implications
Understanding NVU dysfunction in HD opens new avenues for therapeutic intervention. Targeting the components of the NVU to restore phase coherence could potentially alleviate some of the neurodegenerative processes in HD. Potential strategies include:
- Pharmacological Interventions: Developing drugs that enhance endothelial function, astrocyte activity, or pericyte viability may improve NVU phase coherence.
- Anti-inflammatory Therapies: Reducing neuroinflammation may mitigate NVU impairment and slow disease progression.
- Gene Therapy: Correcting the genetic mutation at the root of HD could prevent NVU dysfunction and restore normal NVC.
Conclusion
Reduced phase coherence in the neurovascular unit is a significant contributor to Huntington’s disease pathology. Addressing this aspect of HD could lead to novel therapeutic strategies aimed at preserving neurovascular function and slowing disease progression. Further research is needed to fully elucidate the mechanisms of NVU impairment and develop effective treatments.
DOI: The phase coherence of the neurovascular unit is reduced in Huntington’s disease, Brain Communications (2024). DOI: 10.1093/braincomms/fcae166
References
- Saeedi, S. et al. (2020). Endothelial cell function and dysfunction in neurodegenerative diseases. Frontiers in Neuroscience, 14, 1-13.
- Sweeney, M. D., Ayyadurai, S., & Zlokovic, B. V. (2016). Pericytes of the neurovascular unit: key functions and signaling pathways. Nature Neuroscience, 19(6), 771-783.
- Muoio, V., Persson, P. B., & Sendeski, M. M. (2014). The neurovascular unit – concept review. Acta Physiologica, 210(4), 790-798.
- Montagne, A. et al. (2017). Blood-brain barrier breakdown in the aging human hippocampus. Neuron, 85(2), 296-302.
- Jiang, Q. et al. (2018). Impaired neurovascular coupling in a transgenic mouse model of Alzheimer’s disease. PLoS ONE, 13(2), e0190727.
- Chang, C. C. et al. (2018). Astrocyte-derived VEGF and impaired neurovascular coupling in Huntington’s disease. Annals of Neurology, 84(5), 725-738.
- Ozturk, G. et al. (2020). Chronic neuroinflammation alters neurovascular unit integrity and function in a mouse model of Alzheimer’s disease. Neurobiology of Disease, 137, 104780.
- Lin, A. L. et al. (2014). Neurovascular coupling impairment in mice with amyloid pathology. Journal of Cerebral Blood Flow & Metabolism, 34(4), 568-575.
- Attwell, D. et al. (2010). Glial and neuronal control of brain blood flow. Nature, 468(7321), 232-243.
- Iadecola, C. (2017). The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron, 96(1), 17-42.
- Tong, X. K., Nicolakakis, N., & Hamel, E. (2005). Brain eNOS overexpression restores blood flow response and memory in Alzheimer’s disease. Journal of Cerebral Blood Flow & Metabolism, 25(2), 149-160.
Internal Brain Article References
Brain Exercise to Improve Cognition and Mental Health
Memotenz Review: A Deep Dive into This Popular Nootropic Supplement
SuperBeets Memory and Focus Review: Hype or Help?
Neuriva Plus for a More Focused, More Productive You
Brain Hack or Brain Snack? Our No-Nonsense Review of Onnit Alpha Brain
New Research Shows ‘Profound’ Link Between Dietary Choices and Brain Health

Huntington’s Disease runs in my family. My grandmother had it. Of her four sons it killed three of them.
Only her oldest son, my father, had children and we were born before the test was available and before she began having symptoms and chorea.
I have been tested and don’t have it. My brother isn’t so lucky…